18 catalytic antibodies detailed description editsRS 1
1. Objectives
a. To understand what are ‘Catalytic Antibodies’, their characteristics and role in biological systems
b. To explore the brief history of Catalytic Antibodies
c. To understand in detail on various catalytic antibodies and different modes of generation
d. Various applications
2. Description
2.1. Catalytic Antibodies:
‘Catalytic antibodies’ (antibody specificity + enzyme’s catalytic power) (CatAbs),are immuno-modulators which can increase certain metabolic, physiological and chemical reactions in the body. This is made possible by binding to a chemical group.CatAbs are produced by combining an antibody to a hapten molecule for improving the immunogenicity. The carrier haptens are usually so designed that they resemble the transition state of metabolic reactions, which also evoke a specific response in the organism.Normally larger molecules can effectively elicit antibodies via immunization and hence prior to the actual immunization, small-molecule haptens are mostly attached to a larger protein molecule, called carrier proteins.
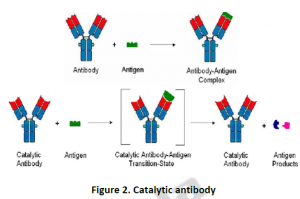
Antibody molecules normally are known for its specificity for binding to its antigen through paratope- epitope interaction; normally they bind simply and are not involved in catalysis of any biochemical reactions. However, when animals are immunized with carrier molecules, CatAbs are produced. The carrier molecules are specially designed to elicit antibodies that have binding pockets which are capable of catalyzing chemical reactions; endorsing an enzymatic nature by lowering a reaction’s activation energy barrier. This is only possible when we design antibodies having a site of binding which is complementary to the transition state, both in terms of charge distribution and 3D structure. This complementarity encourages the substrate to adopt a transition-state-like geometry and charge distribution, increase the reaction selectivity, thus leading to catalysis. On contrary to enzymes, one of the most interesting features of CatAbs is that the desired reaction selectivity can be engineered into the antibody using a carrier hapten, which has been appropriately designed. CatAbs demonstrate a high degree of substrate selectivity as well as specificity. Furthermore, CatAbs that have region selectivity needed to produce a single specific product for a reaction have been produced. In such cases, other products are normally observed in the absence of the antibody.
2.2. Characteristics of catalytic antibodies:
- CatAbscan either be present naturally (innate, in case of autoimmune diseases) or be elicited by vaccination or immunization. Catalytic antibodies utilize – antibody’s specificity & enzyme’s catalytic power
- The mode of action include nucleophilic catalysis, coordination with metal ions, induction of conformational strain and stabilization of transition states
- Haptens: The term ‘hapten’ derived from a Greek word ‘Haptein’ means to fasten. Small molecules that stimulates the production of antibodies are often conjugated to larger molecule, called a carrier molecule or haptens.
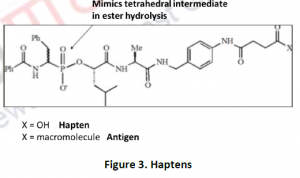
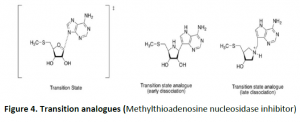
- Transition state analogs:Enzyme does catalysis by maintaining the middle state; known as the transition state and certain chemical compounds resembles the transition state of a substrate by its chemical structure and are known as transition state analogs.In general, for CatAbs compounds are synthesized with closer similarity towards transition state other than the substrate itself and hence will be highly specific and potent inhibitors of enzymes.
2.3.History of catalytic antibodies
- In 1948, Linus Pauling an American biochemistexplained the principle of enzyme catalysis in detail. He proposed that compounds resembling the middle state or transition state of an enzyme reaction will be very potent inhibitors of those enzymes and hence these mimics were called transition- analogs.
- In 1969Jenks, suggested that one could obtain antibodies with catalytic activity by generating antibodies raised against a stable analogue of the transition state.
- In 1986, Peter Schultz and Richard Lernergeneratedabzymeswhich catalyze ester hydrolysis for the first time.
- In 1989Paul et al., discovered the first natural catalytic antibodies (IgG) which hydrolyze the vasoactive intestinal peptide (VIP)in bronchial asthmatics.This discovery was followed by IgG (DNase activity) and IgG (RNase activity) in systemic lupus erythematosus (SLE) and in several autoimmune (AI), viral disorders etc
2.4. Structure and mechanism of action of a catalytic antibody
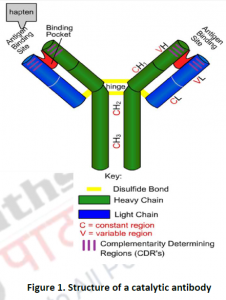
Structurally, CatAbs are similar to “Y-shaped” Ig G-type (Figure 1) which consists of two identical heterodimers linked by S-S bonds. Each heterodimer composed of a light chain and a heavy chain. One end of the immunoglobulin moleculeconsist of conserved domains (Fc) which are constant regions formed in between two heavy chains. Conserved domains have been found to have similar amino acid compositions in most immunoglobulins, other than the epitope-paratope interaction that varies. The opposite end is the variable domains (Fv), responsible for specifically binding antigen. A single deep pocket (antigen binding site) exists in the interface of two of the VHand VLregions. “Hot spots” within the variable domains, which are vital for antigen specificities, are called complementarity determining regions (CDRs). The amino acids within the hot spots interact with the antigen through non-covalent interactions.
CatAbs are known to bind very tightly to the transition state analog haptens, which have been used to produce them during the process of immunization. Within the antibody binding sites, the binding forces are required to provide stability to the transition states and intermediates, thus lowering the reaction’s overall energy barrier and increasing the reaction rate. Rate of reaction catalyzed by a CatAb is measured by kinetic parameters, such as, Km and Vmax. Catalytic antibodies are characterized by a low KM, indicating their higher affinity to bind a target molecule. On the other hand, the CatABs have low Vmax values, indicating a slower reaction rate and hence still unable to approach even near to the rates of reaction catalyzed by biological enzymes. Additionally, CatAbs adopts an “induced fit” binding mode which leads to an enhanced complementarity between the antibody combining site and the hapten. This is in contrast to real enzymes which are able to typically change their own conformation in binding the transition state.
2.5. Construction & generation of catalytic antibodies
An artificial catalytic antibody designed as per need is known as ‘Abzyme’. It’s usually a monoclonal antibody raised in lab, against specific synthetic haptens, where they specificially bind and catalyze a reaction. The major components for Abzyme generation are:
- A transition state analogue which is able to mimic the transition state of desired reaction is synthesized. These are called as haptens
· Haptens are attached to carrier molecule which is capable of eliciting an antibody response.
These are called as antigens
· Specific antigen designed is injected to invivo models like mouse or rabbit
· Monoclonal antibodies are isolated and purified
- These monoclonal antibodies are further tested for catalytic activity by cat ELISA, HPLC etc