11 Enzyme Inhibition
1. Objectives
- Enzyme inhibition
- Various types of enzyme inhibitions
2. Description – Enzyme Inhibition
An enzyme catalyzed reaction can be hindered or reduced by a number of substances. Some others like urea are known as denaturants, being non-specific in their mode of action.But if any compound act in a fairly specific way in inhibiting the catalysis of a particular enzyme they are called inhibitors. The loss in activity can either be of two types-
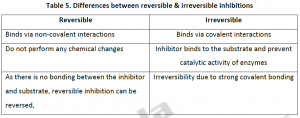
- Reversible, where the activity can be restored by the removal of the inhibiting compound.It’s temporary.
- Irreversible, where the loss of activity cannot be recovered within the stipulated time of interest. It is permanent. Irreversible inhibition behave as time dependent loss of enzyme concentration with lowered Vmax or incomplete in activation with time dependent change in both Km and Vmax.
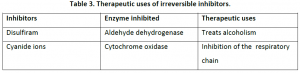
Heavy metal ions like mercury, lead etc cause irreversible inhibition, which bind strongly to the aminoacid backbone termed as “suicide inhibition”.
There are several reasons behind the need for studying enzyme inhibition mechanisms. They are:-
- Exploring potential mechanisms in multi-substrate reactions.
- Studying the relative binding affinity of competitive inhibitors to the enzyme active site, in the absence of 3-D structure information.
- For understanding various control mechanisms-how the balance of protease enzymes and their inhibitors in tissues achieve homeostasis.
- For various commercial applications like pesticide,insecticide,weed- killers,pharmaceutical compounds like drugs etc.
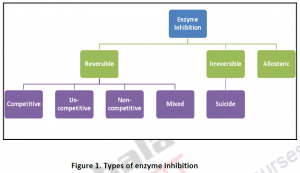
2.1. Reversible Inhibition:
Figure 1. Types of enzyme inhibition
In this type of inhibition, the hindrance is temporary and thus non covalent interactions like hydrogen bonds,ionic bonds or hydrophobic bonds form between inhibitors and the enzyme. Even though these are weak bonds,multiple such bonds cause strong and specific binding. Despite absence of any chemical reactions,the inhibitors can easily remove or exchange by dilution or dialysis. After removing the inhibitor,enzyme can be fully restored in reversible inhibition. Equilibrium is established between free inhibitor and enzyme-inhibitor [EI] complex (Figure 2).
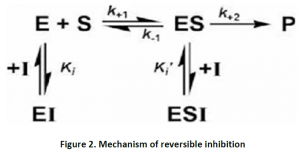
Reversible inhibitions areof different types. The classification is based according to the effect of varying the concentration of the enzyme’s substrate on the inhibitor.
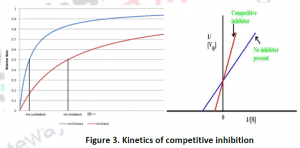
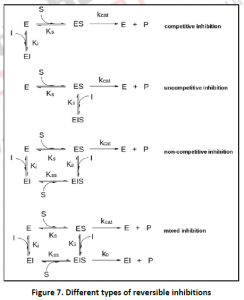
2.1.1. Competitive Inhibition: In this type of reversible inhibition, both the substrate and its inhibitor cannot bind to the enzyme at the same time to the all osteric/active site. This normally occurs due to the structural similarity of substrate and the inhibitor,which results with affinity for the active site.The inhibition can be recovered by the presence of high concentration of substrate,out coming the competing inhibitor. Vmax of the reaction is unchanged, while kd, the dissociation constant is apparently increased. Competitive inhibitors can also be used to find the enzyme active site.
Eg: N-(phosphonacetyl)-L-asparate also known as PALA is a competitive inhibitor for asparatetranscarbamoylose.
Eg: Malonate is a competitive inhibitor of enzyme succinate dehydrogenase,and competes with succinate.
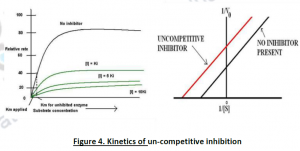
2.1.1. Uncompetitive Inhibition: It’s an anti-competitive inhibition; where the inhibitor binds only to the substrate-enzyme complex. According to its kinetics,Vmax and Km decrease. This type of inhibition works best in case of high concentration of the substrate. The substrate and the uncompetitive inhibitor does not resemble each other. Eg:-Lithium and phosphoinositide cycle.
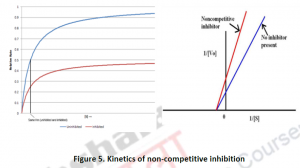
2.1.3. Non-competitive Inhibition: A non-competitive inhibitor is one which reacts with enzyme-substrate or [ES] complex. It does not affect the binding of the substrate, but slows down the reaction rate for formation of the enzyme-product [EP] complex. The only factor on which the extent of hindrance or inhibition depends is the inhibitor concentration. There will be a decrease in Vmax but km will remain the same.
Eg: Alanine non competitively inhibits the enzyme pyruvate kinase.
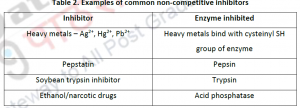
2.1.4. Mixed Inhibition: In this type of inhibitor, inhibitor is capable of binding to both free enzyme as well as enzyme- substrate complex. In this case, Vmax and Kmax varies. Mixed inhibitor binds to the all osteric site. This type of inhibition cannot overcome by increasing substrate concentration S,but can be reduced. The inhibitor binding to the all osteric site changes the structural confirmation to reduce the affinity of the substrate. Eg: Mixed inhibition is observed on case of oxidoreductase activity of xanthine.
oxidase by Pd2+ ion
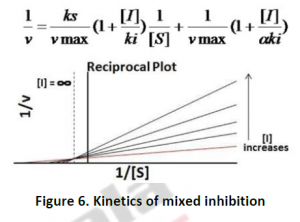
2.2. Irreversible Inhibition:In this type of inhibition, the hindrance is of permanent nature by modifying enzyme covalently. These types of inhibitors often contain electrophilic functional groups like fluorophosphates, aldehydes, haloalkanes, alkenes, nitrogen mustards, phenyl sulfonates, Michael acceptors etc, which react with aminoacid sidechains having nucleophilic residues.
These inhibitors are very specific in the mechanism of inactivation for a particular class of enzyme-They do irreversible inhibition by specially altering the active site. They display inhibition which is time-dependent. Their potency cannot be characterized by IC50 value. These inhibitors increase Kmand decrease Vmax.
Eg: Diisopropylflurophosphate (DFP) is an irreversible protease inhibitor.
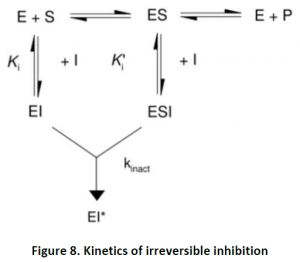
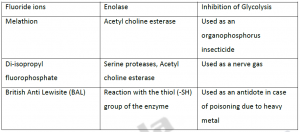
Suicide inhibition- This is another type of irreversible inhibition. In this case, the target enzyme converts the inhibitor compound into a reactive form in its active site. They are also known as mechanism based inhibitors or transition state analogs.
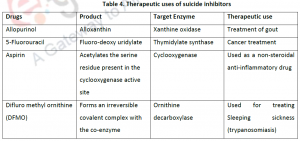
Eg:- DFMO [α-difluromethyl ornithine], an analogue of ornithine inhibits ornithine decarboxylase.
Eg:- Allopurinol is a suicide inhibitor of xanthine oxidase Aspirin inhibits cyclooxygenase.
2.3. Allosteric Inhibition: Allosteric inhibition is a type of enzyme regulation, in which allosteric inhibitor binds to a site other than the active site of the enzyme. This additional site to which effector binds is called allosteric site. When these effectors bind to the protein, results with conformational change and cause enhancement in activity is known as allosteric activators. When they decrease the activity of the protein, they are known as allosteric inhibitors. Allosteric enzymes are K or V types.
Models of allosteric regulation: The allosteric effects or mechanism is well described by the concerted HWC model, which was put forth by Monod, Wyman and Changeux. Another model called the sequential model, proposed by Koshland, Nemethy and Filmer, also possibly explains the allosteric regulation. Both these models postulate that enzyme subunits exist in one two conformations – tensed (T) or relaxed(R) states.
2.3.1 Concerted model: This model is known as symmetry or MWC model. According to this model, enzyme sub units exist in same conformation, they are connected and a slight conformational change in any one of the sub units is conferred to all other sub units of the enzyme. When any ligand or substrate is absent, the equilibrium favors towards either of the conformational states. Among the tensed and relaxed states, the ‘R’ state has higher affinity than ‘T’ state. The most successful application of this model is this regulation of hemoglobin.
2.3.2. Sequential model: In contrary to concert model, sequential model states that enzymes subunits are not connected, such that any change in the enzyme conformation leads to induction of a similar change in the others. When a subunit randomly collides with substrate, an induced fit converts a subunit from the ‘T’ state to ‘R’ state.
2.3.2. Morpheein model: The third model is a dissociative concerted model known as morpheein model. This is physiologically significant homo-oligomeric tetramer structure. Transition in the morpheein model are assisted by dissociation of oligomer , conformational change in dissociated state and reassembly of oligomers. So far, one of the best characterized morpheein is the enzyme porphobilinogen synthase.
Types of allosteric regulation:
There are mainly two types of allosteric regulation. Homotropic & Heterotropic.
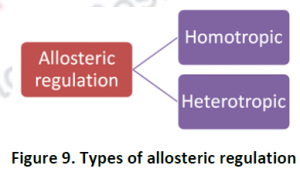
- Homotropic regulation: It’s a positive modulation- The modulator acts not only as a substrate but also as a regulatory molecule of the target enzyme.
Eg: CO2 is a heterotropic modulator of haemoglobin. - Heterotropic regulationIt can either be a positive or negative modulation. Here the modulator is a regulatory molecule but not an enzymes substrate.
Eg:CO2is a heterotropic modulator of haemoglobin.
3. Importance of enzyme inhibition:
- Understanding regulation of enzyme activity in living cells
- Elucidation of the cellular metabolic pathways by accumulation of intermediates Helps in identification o
- catalytic or functional groups present at the enzyme active site
- Helps in providing information on enzyme’s substrate specificity
- Helps in studying the mechanism of catalytic activity
- Competitive or suicide inhibitors also find therapeutic applications.
Conclusion
Specific inhibition of target enzyme has been attributed through various modes of inhibition. It can either be reversible, irreversible or all osteric model of inhibitions. Other than the models and mechanisms of inhibitions, they have immense applications.
| you can view video on Enzyme inhibition |
